Real-World Evidence Supports Clinical Effectiveness of neffy® (epinephrine nasal spray) in Patients Experiencing Anaphylaxis
Findings accepted for publication in the official journal of the American College of Allergy, Asthma and Immunology
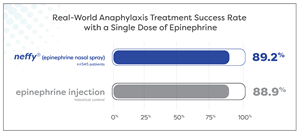
About 9 out of every 10 patients experiencing anaphylaxis were effectively treated with a single dose of neffy
Results suggest real-world effectiveness of neffy is consistent with that historically reported for epinephrine injection
SAN DIEGO, Sept. 08, 2025 (GLOBE NEWSWIRE) -- ARS Pharmaceuticals, Inc. (Nasdaq: SPRY), a commercial stage biopharmaceutical company dedicated to empowering at-risk patients and their caregivers to better protect patients from allergic reactions that could lead to anaphylaxis, today shares real-world evidence evaluating the clinical performance of neffy® (epinephrine nasal spray) in patients experiencing anaphylaxis symptoms during oral food challenge and allergen immunotherapy. These findings represent the first large-scale analysis of treatment outcomes with neffy during routine clinical practice and was accepted in August for publishing as a correspondence in the Annals of Allergy, Asthma and Immunology, the official journal of the American College of Allergy, Asthma and Immunology.
Nearly 90% (89.2%) of 545 patients experiencing anaphylaxis symptoms during oral food challenge and allergen immunotherapy were successfully treated with a single dose of neffy by a healthcare provider. Meta-analyses report a similar proportion of patients, 88.9%, being successfully treated with a single dose of epinephrine intramuscular injection or auto-injector by a healthcare provider for food-induced anaphylaxis.1 This highly similar treatment success rate supports that the real-world clinical effectiveness of neffy in anaphylaxis is consistent with epinephrine injection.
Importantly, these real-world data build upon previously published clinical evidence, including a prospective Phase 3 study2 (n = 15 patients) assessing the efficacy of neffy for the treatment of oral food challenge-induced anaphylaxis symptoms. In that study, no patients required a second dose of neffy for treatment of the initial anaphylactic reaction.
“These data reinforce existing findings and is the first large-scale report of real-world treatment outcomes with neffy during anaphylaxis events. The finding that about 9 out of every 10 patients were successfully treated with a single dose of neffy in more than 500 patients is essentially identical to the historic response rates observed with epinephrine injection,” said Dr. Thomas B. Casale, M.D., Professor of Medicine and Pediatrics and Chief of Clinical and Translational Research in the USF Health Morsani College of Medicine's Division of Allergy and Immunology at the University of South Florida in Tampa, Florida. “We believe these real-world outcomes data support the clinical interchangeability of neffy and epinephrine injection, building on the clinical studies conducted for FDA approval that showed neffy achieved blood levels and pharmacodynamic responses within the range of approved injection products.”
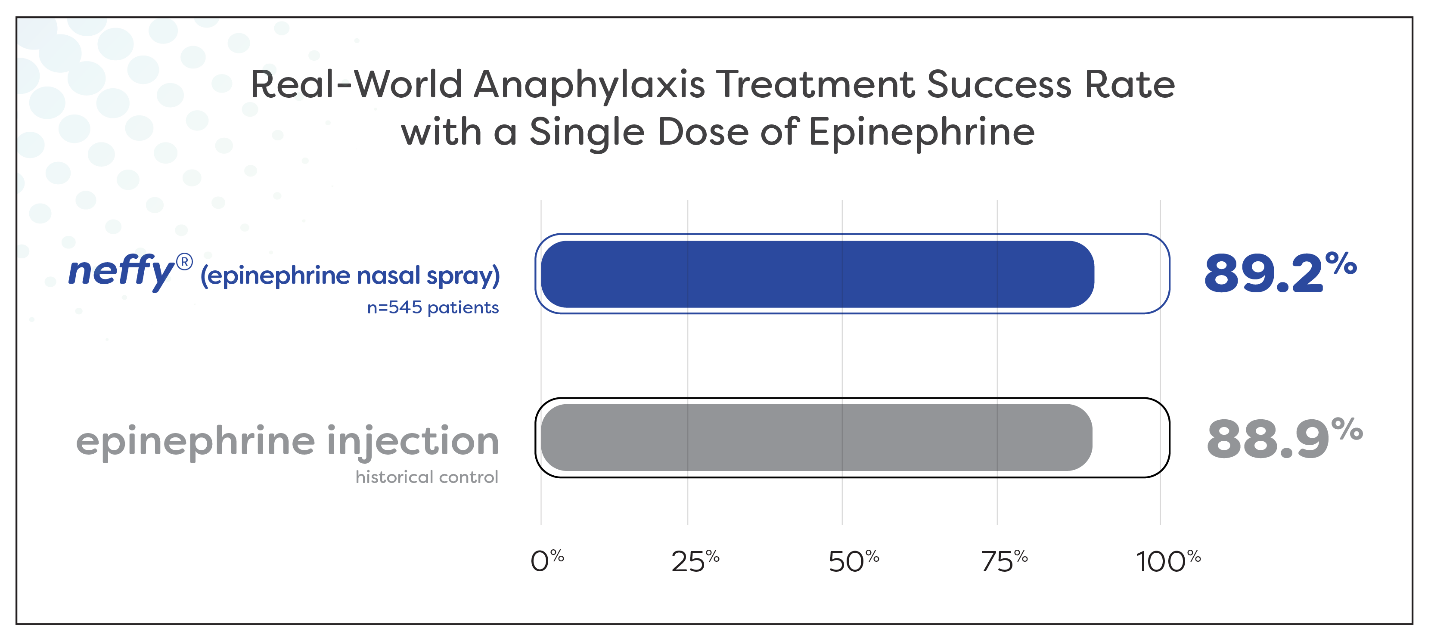
Figure 1: Treatment success rate with a single dose of neffy administered by a healthcare provider during oral food challenge and allergen immunotherapy during the neffy experience program (n = 545 patients) compared to historic control treatment success rate with a single dose of epinephrine injection administered by a healthcare provider during food-induced anaphylaxis as reported by Patel et. al. JACI, 2021 (n = 12,615 patients).
About the neffy Experience Program
The data from this retrospective observational analysis was collected from healthcare providers participating in the neffy experience program. In the neffy experience program, healthcare providers were given six doses of neffy for use to rescue patients experiencing anaphylaxis symptoms during oral food challenge or allergen immunotherapy. neffy labeling states that a second dose should be administered if anaphylaxis symptoms continue or get worse starting 5 minutes after the first dose.
As of the March 2025 data cut-off, 301 healthcare providers had responded to the survey instrument, and a total of 545 patients were reported having been treated with neffy 2 mg. Four hundred and eighty-six (486) of these patients had been successfully treated with a single dose of neffy 2 mg, with the remaining 59 patients requiring a second dose of epinephrine. The neffy experience program is actively ongoing and now includes both the 2 mg and 1 mg doses.
This data from the neffy experience program is consistent with clinical trials recently published on the real-world clinical effectiveness of neffy. However, this observational analysis differs from a randomized clinical trial in several ways. The studies have different endpoints and there are inherent limitations in real-world observational studies, including lack of randomization, lack of uniform timing or type of clinical assessments and challenges with missing data.
About neffy®
neffy is a nasal spray used for emergency treatment of allergic reactions including anaphylaxis, in adults and children aged 4 years and older who weigh 33 lbs. or greater.
INDICATION AND IMPORTANT SAFETY INFORMATION FOR neffy (epinephrine nasal spray)
INDICATION
neffy is indicated for emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients aged 4 years and older who weigh 33 lbs. or greater.
IMPORTANT SAFETY INFORMATION
neffy contains epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life-threatening, can happen in minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or other unknown causes.
Always carry two neffy nasal sprays with you because you may not know when anaphylaxis may happen and because you may need a second dose of neffy if symptoms continue or come back. Each neffy contains a single dose of epinephrine. neffy is for use in the nose only.
Use neffy right away, as soon as you notice symptoms of an allergic reaction. If symptoms continue or get worse after the first dose of neffy, a second dose is needed. If needed, administer a second dose using a new neffy in the same nostril starting 5 minutes after the first dose. Get emergency medical help for further treatment of the allergic emergency (anaphylaxis), if needed after using neffy.
Tell your healthcare provider if you have underlying structural or anatomical nasal conditions, about all the medicines you take, and about all your medical conditions, especially if you have heart problems, kidney problems, low potassium in your blood, Parkinson’s disease, thyroid problems, high blood pressure, diabetes, are pregnant or plan to become pregnant, or plan to breastfeed.
Tell your healthcare provider if you take or use other nasal sprays or water pills (diuretics) or if you take medicines to treat depression, abnormal heart beats, Parkinson’s disease, heart disease, thyroid disease, medicines used in labor, and medicines to treat allergies. neffy and other medications may affect each other, causing side effects. neffy may affect the way other medicines work, and other medicines may affect how neffy works.
neffy may cause serious side effects. If you have certain medical conditions or take certain medicines, your condition may get worse, or you may have more or longer lasting side effects when you use neffy.
Common side effects of neffy include: nasal discomfort, headache, throat irritation, chest and nasal congestion, feeling overly excited, nervous or anxious, nose bleed, nose pain, sneezing, runny nose, dry nose or throat, tingling sensation, including in the nose, feeling tired, dizziness, nausea, and vomiting.
Tell your healthcare provider if you have any side effects that bother you or that do not go away after using neffy.
These are not all of the possible side effects of neffy. Call your healthcare provider for medical advice about side effects. To report side effects, contact ARS Pharmaceuticals Operations, Inc. at 1-877-MY-NEFFY (877-696-3339) or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see the full Prescribing Information and Patient Information for neffy.
About Type I Allergic Reactions Including Anaphylaxis
Type I allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only FDA-approved medication for these reactions. While epinephrine auto-injectors have been shown to be highly effective, there are well published limitations that result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. There are approximately 40 million people in the United States who experience Type I allergic reactions. Of this group, over the last three years, approximately 20 million people have been diagnosed and treated for severe Type I allergic reactions that may lead to anaphylaxis, but (in 2023, for example) only 3.2 million filled their active epinephrine auto-injector prescription, and of those, only half consistently carry their prescribed auto-injector. Even if patients or caregivers carry an auto-injector, more than half either delay or do not administer the device when needed in an emergency.
About ARS Pharmaceuticals, Inc.
ARS Pharma is a biopharmaceutical company dedicated to empowering at-risk patients and their caregivers to better protect patients from allergic reactions that could lead to anaphylaxis. The Company is commercializing neffy® (trade name EURneffy® in the EU), an epinephrine nasal spray indicated in the U.S. for emergency treatment of Type I allergic reactions, including anaphylaxis, in adult patients and pediatric patients 4 years of age and older who weigh 33 lbs. or greater, and in the EU for emergency treatment of allergic reactions (anaphylaxis) due to insect stings or bites, foods, medicinal products, and other allergens as well as idiopathic or exercise induced anaphylaxis in adults and children who weigh 30 kg or greater. For more information, visit www.ars-pharma.com.
Forward Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to: expectations and evaluations regarding the clinical performance and patient benefits of neffy, including its needle-free, portable and easy to use design; the belief that real-world outcomes data support the clinical interchangeability of neffy and epinephrine injection; and the beliefs that such data will continue to build on the clinical studies of neffy conducted for FDA approval that showed neffy achieved blood levels and pharmacodynamic responses within the range of approved epinephrine injection products and on previously published clinical evidence, including a prospective Phase 3 study assessing the efficacy of neffy for the treatment of oral food challenge-induced anaphylaxis symptoms; and other statements that are not historical fact. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “anticipate,” “believe,” “can,” “could,” “expect,” “if,” “may,” “potential,” “plan,” “will,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharma’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation: potential safety and other complications from neffy; the ability to maintain regulatory approval for neffy in its currently approved indications; the scope, progress and expansion of developing and commercializing neffy; the scope, progress and expansion of developing our intranasal epinephrine technology; clinical trial results; the potential for governments and payors to delay, limit or deny coverage for neffy; the size and growth of the market for neffy and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; ARS Pharma’s ability to protect its intellectual property position; and the impact of government laws, regulations and policies. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, filed with the Securities and Exchange Commission (“SEC”) on May 14, 2025 and in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, filed with the SEC on August 13, 2025. These documents can also be accessed on ARS Pharma’s website at www.ars-pharma.com by clicking on the link “Financials & Filings” under the “Investors & Media” tab.
The forward-looking statements included in this press release are made only as of the date hereof. ARS Pharma assumes no obligation and does not intend to update these forward-looking statements, except as required by law. For more information, visit www.ars-pharma.com, and follow us on LinkedIn and X.
Investor Contact:
Justin Chakma, ARS Pharma
justinc@ars-pharma.com
Media Contact:
Christy Curran, Sam Brown Inc.
christycurran@sambrown.com
615.414.8668
References
1 Patel N, Chong KW, Yip AYG, Ierodiakonou D, Barta J, Boyle RJ, et al. Use of multiple epinephrine doses in anaphylaxis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2021;148(5):1307-1315.
2 Ebisawa M, Takahashi K, Takahashi KK, Yanagida N, Sato S, Lieberman J, et al. Epinephrine nasal spray improves allergic symptoms in patients undergoing oral food challenge, phase 3 trial. J Allergy Clin Immunol. 2025; doi:10.1016/j.jaip.2025.06.038.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/890e02e2-e6bc-4c22-a1cf-a5b0a47fc286

Figure 1
Treatment success rate with a single dose of neffy administered by a healthcare provider during oral food challenge and allergen immunotherapy during the neffy experience program (n = 545 patients) compared to historic control treatment success rate with a single dose of epinephrine injection administered by a healthcare provider during food-induced anaphylaxis as reported by Patel et. al. JACI, 2021 (n = 12,615 patients).
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.